The majority of paranormal investigators would readily agree that ghosts must be some form of energy. As such, it is important to understand many of the laws that govern energy. Energy exists in many forms, such as heat, light, chemical energy, and electrical energy. Energy is the ability to bring about change or to do work. Thermodynamics is the study of energy.
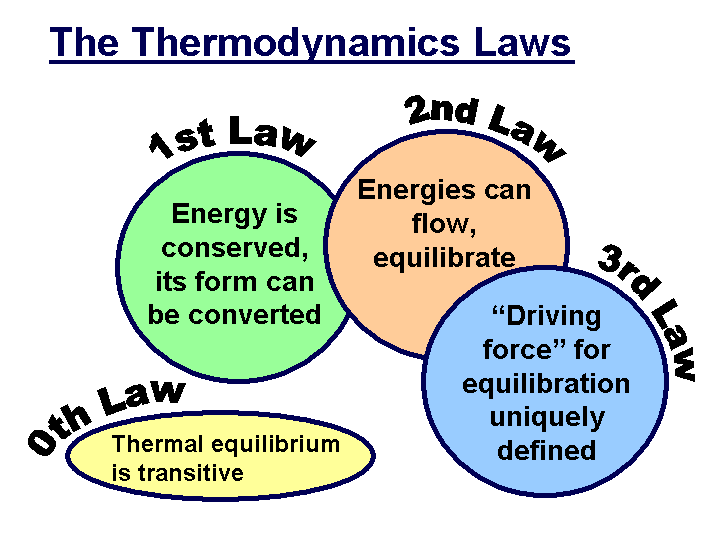
First Law of Thermodynamics: Energy can be changed from one form to another, but it cannot be created or destroyed. The total amount of energy and matter in the Universe remains constant, merely changing from one form to another. The First Law of Thermodynamics (Conservation) states that energy is always conserved; it cannot be created or destroyed. In essence, energy can be converted from one form into another. This is the most cited law for the existence of ghosts used by many paranormal researchers today.
The Second Law of Thermodynamics states that "in all energy exchanges, if no energy enters or leaves the system, the potential energy of the state will always be less than that of the initial state." This is also commonly referred to as entropy. In the process of energy transfer, some energy will dissipate as heat. Entropy is a measure of disorder: cells are NOT disordered and so have low entropy. The flow of energy maintains order and life. Entropy wins when organisms cease to take in energy and die. If ghosts do exist, then under this law their energy would be less than what it was when it was in a living body. When you research the energetic possibilities of the human body, you’ll discover that this really isn’t that much energy.
The Third Law of Thermodynamics: The third law of thermodynamics states that as a system approaches absolute zero of temperature all processes cease and the entropy of the system approaches a minimum value or zero for the case of a perfect crystalline substance.
The British scientist and author C.P. Snow had an excellent way of remembering the three laws:
- You cannot win (that is, you cannot get something for nothing, because matter and energy are conserved).
- You cannot break even (you cannot return to the same energy state, because there is always an increase in disorder; entropy always increases).
- You cannot get out of the game (because absolute zero is unattainable).
Sources:
- Sachs, M. 1988. Einstein versus Bohr: The continuing controversies in physics. La Salle, Ill.: Open Court.
- http://www.encyclopedia.com